Treatment pathway
AAV is a chronic condition with a relapsing course that needs long-term immunosuppressive therapy1–4
EULAR recommendations state that AAV patients should be offered best care which must be based on shared decision making between the patient and the physician considering efficacy, safety and costs5
The balance of treatment
Treatment must balance controlling vasculitis with minimising treatment-related damage1–4
At the time of AAV diagnosis or relapse, the major concerns around treatment involve controlling vasculitis activity and minimising the acute adverse effects from therapy.1,2 Later when remission is achieved, cumulative organ damage, often related to long-term low dose glucocorticoids, and patient experience are more important.1–4
The EULAR Guidelines now state that patients should be periodically screened for treatment-related adverse effects and co-morbidities. They recommend prophylaxis and life-style advice to reduce treatment-related complications and other co-morbidities.5
Treatment-related AEs are the leading cause of acute mortality1
In the first year after a GPA or MPA diagnosis, 56 of 524 patients died. A total of 59% of these deaths were as a result of treatment-related AEs.1*
A systematic literature review of glucocorticoid-related AEs in AAV clinical studies published between 1 January 2007 and 30 January 2018 identified mortality (33%) as the glucocorticoid-related serious AE with the highest frequency observed during early induction treatment. Other common glucocorticoid-related serious AEs experienced by patients include infections (20%), musculoskeletal (17%) and renal disorders (15%).2†
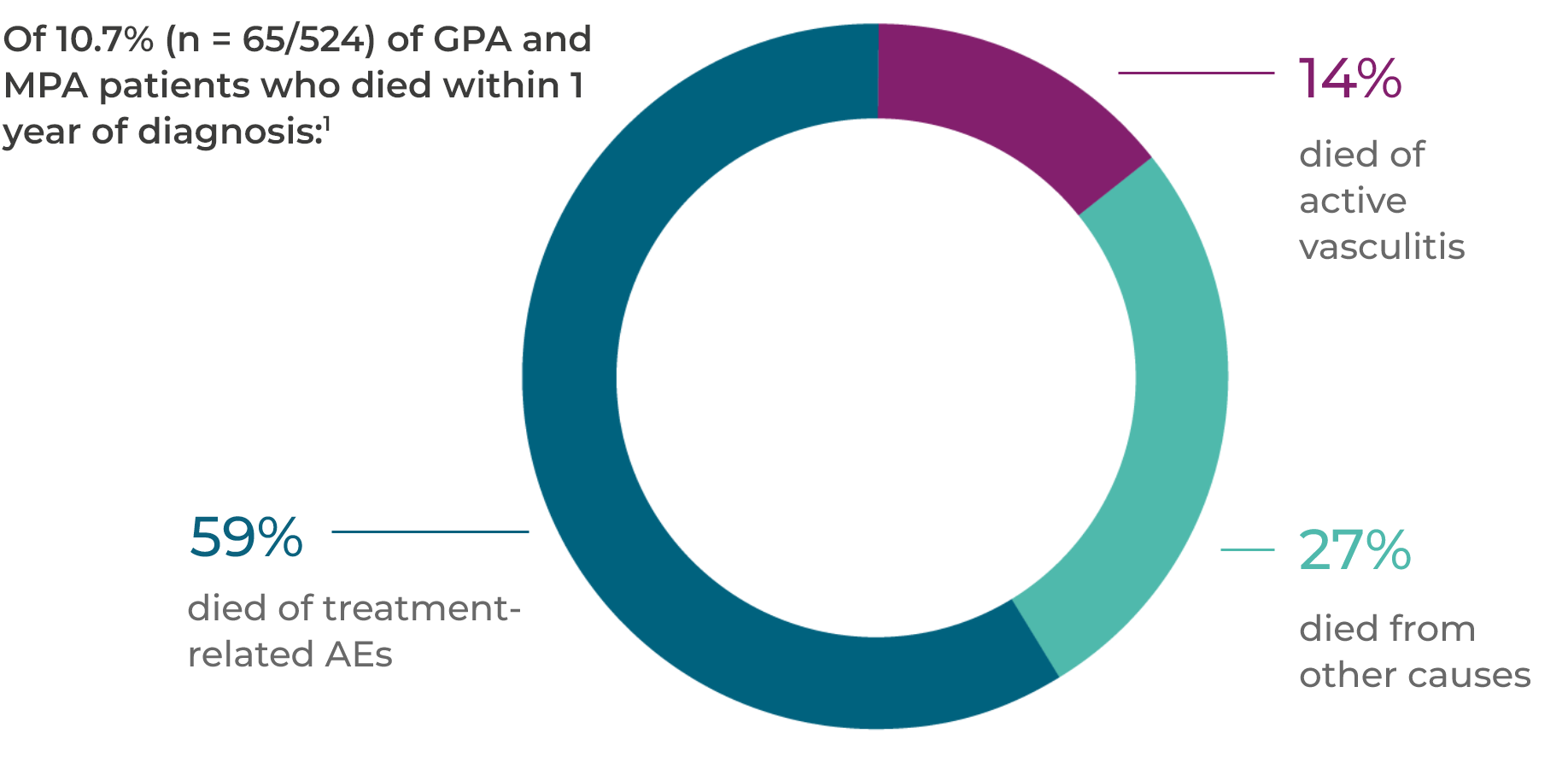
Latest clinical data in AAV
RAVE
Rituximab (RTX) was found to be as effective as cyclophosphamide (CYC) for the induction and maintenance of remission, but remission rates remain variable
2010
RITAZAREM
Preliminary results show that a reduced glucocorticoid (GC) dose can be effective for reinducing remission in AAV. RTX was superior to azathioprine (AZA) at maintaining remission
2014
PEXIVAS
Reducing the GC dose in severe AAV patients did not significantly impact the primary outcome of death or end-stage renal disease, but did reduce the rate of infection (HR=0.69)
2018
ADVOCATE
Avacopan-based regimen is
non-inferior at achieving remission at Week 26 and superior at sustaining remission at Week 52 compared to a GC-based regimen1
2018
MAINRITSAN 1
RTX is more effective than AZA for maintenance of remission, but GC use and relapse remain common
2019
MAINRITSAN 2
Tailored and fixed-schedule RTX regimens are equally as effective at maintaining remission, but relapses still occur
2020
MAINRITSAN 3
Extended maintenance therapy leads to better clinical outcomes
2020
Real-World Experience
Many patients do not achieve or sustain full remission1*
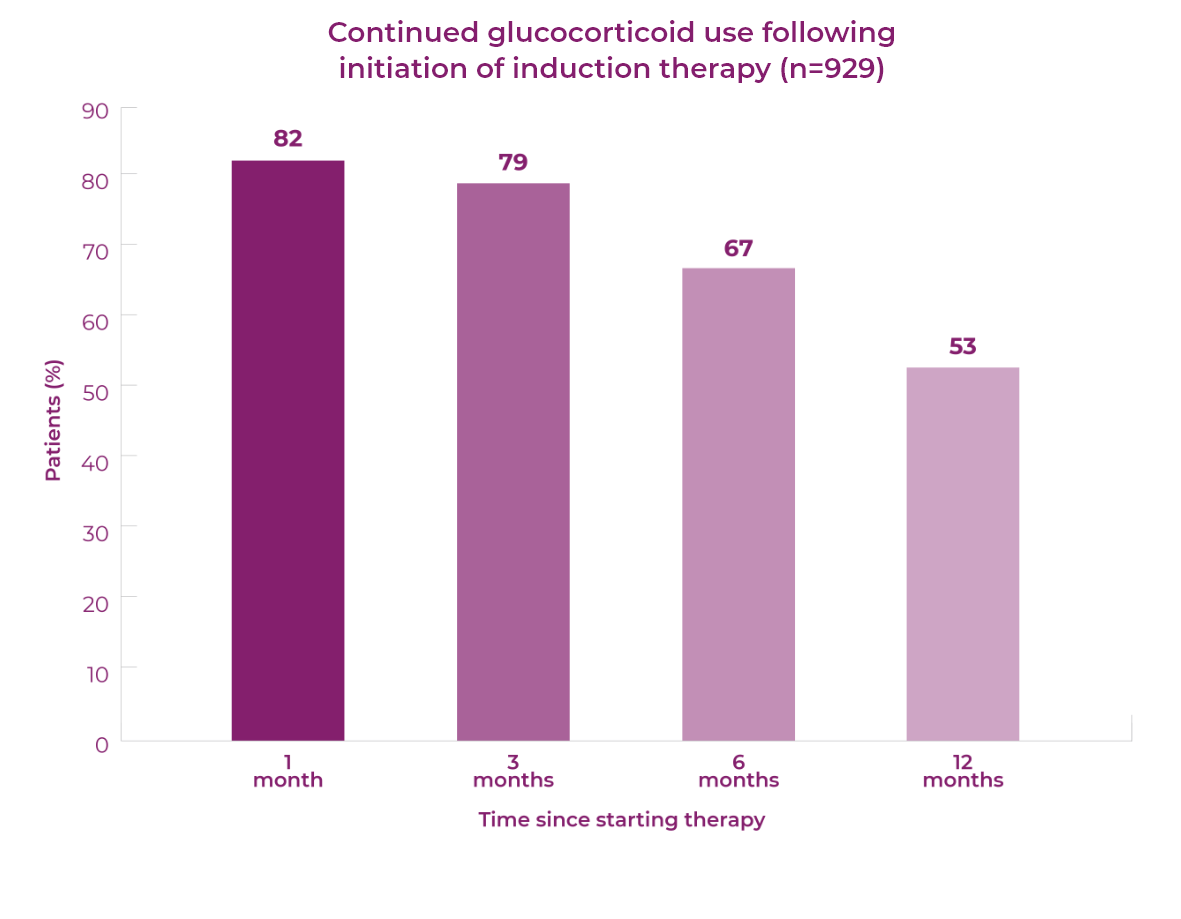
Patient response to remission induction therapy is variable. After 12 months of therapy (n=929), 59% of GPA (granulomatosis with polyangiitis, previously called Wegener’s) and MPA (microscopic polyangiitis) patients achieve full remission, and >50% of patients continue glucocorticoid use.1 More patients need to achieve and sustain remission without prolonged glucocorticoid use.
Response to induction therapy is variable and many patients do not achieve a full response, even at 12 months1
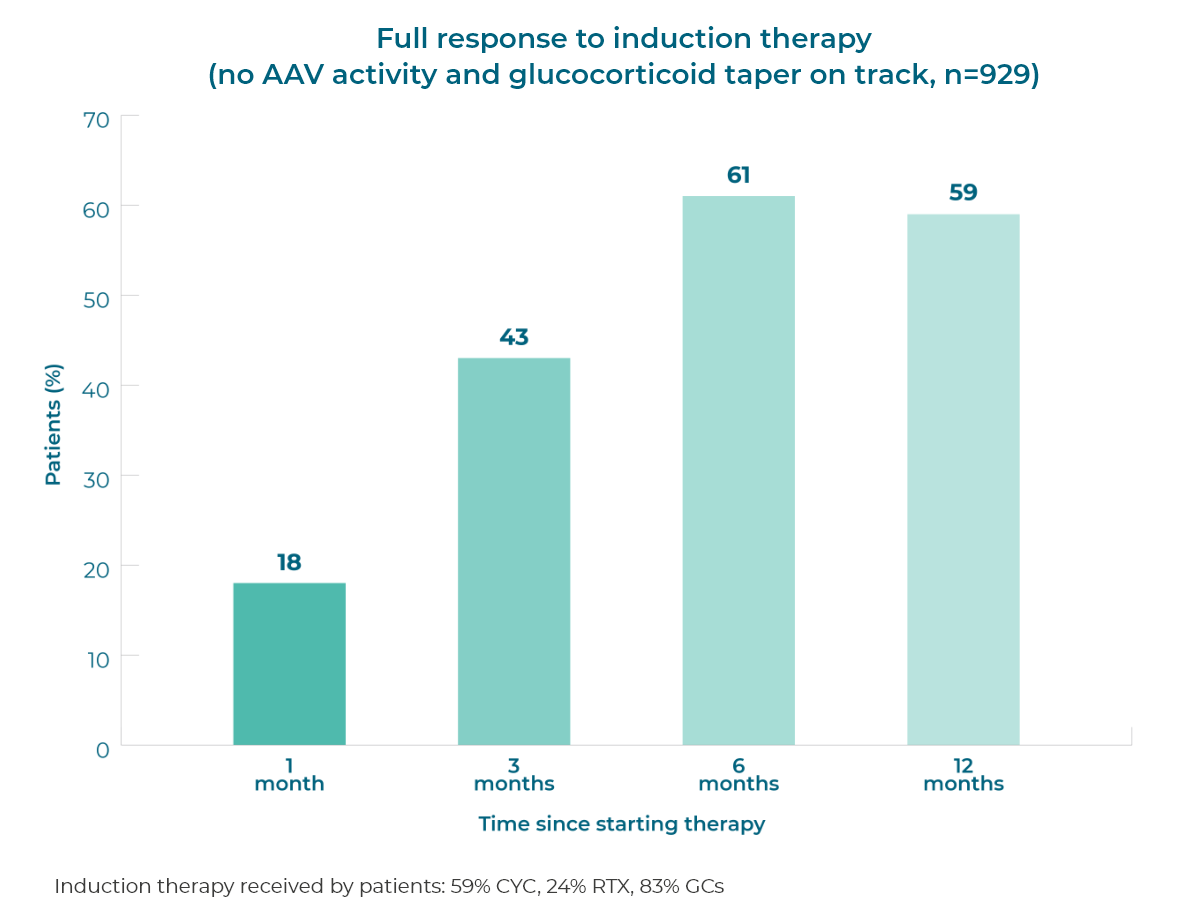
>50% of patients continue to use glucocorticoids 12 months after starting induction therapy1
Real-world treatment data demonstrates that AEs remain an issue for new and relapsing patients1,2
Over the first 12 months of treatment in patients with GPA or MPA, AEs are common.1,2 At this time, glucocorticoid dose is typically highest, as current treatment guidelines recommend the use of glucocorticoids for remission induction.1–3 The majority of patients continue to use glucocorticoids 12 months after starting remission induction.1,2
New patients (n=929)1*




Relapsing patients (n=268)2*





Introduction to AAV
AAV is a rare, severe small vessel vasculitis that affects multiple organs and has a high acute mortality risk1
Read more
Disease mechanism
The interaction between the activated alternative complement pathway, neutrophilsand C5a is at the heart of vasculitic damage in AAV2
Read more